
Mediscribe team provides XEVMPD data management services to help pharmaceutical companies prepare and manage their product information, as well as submission in Eudravigilance database until ISO IDMP implementation.
ISO IDMP Global in scope and provides an internationally accepted frame work for consistent documentation, coding and exchange of product information between global regulators, manufacturers, suppliers and distributors. The scope of product information is significant and does not only include Authorized products but also Investigational Medicinal Products.
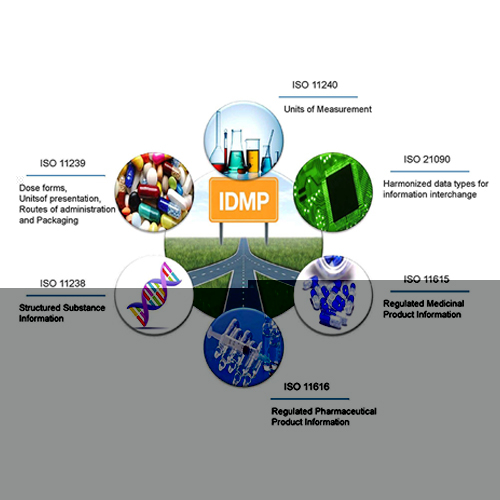
Mediscribe Approach
Mediscribe Pharmacovigilance Specialists have a standard approach to help our clients prepare for the upcoming challenges with IDMP. The approach contain a gap analysis to identify the individual data gaps and define important data elements and structures, which help to plan the needed changes in the organization in order to become IDMP compliant.




